Download & Install
Darasa Huru App
DOWNLOAD
Structure of Matter
State of matter is defined in terms of the phase transitions which indicate the change in structure and properties. Solids, liquids and gases all are made up of microscopic particles. The behavior of all these particles also varies in three phases.
The Concept of Matter
Explain the concept of matter
Matter is anything, such as a solid, liquid or gas, that has weight (mass) and occupies space. For anything to occupy space, it must have volume.
The Particular Nature of Matter
Justify the particulate nature of matter
Matter
is made up of tiny particles. The particles are atom or molecules,
examples of substances, which are made up of atoms, are: gold, copper,
Argon and silver; and those made up of molecules includes oxygen, water
and ammonia.
is made up of tiny particles. The particles are atom or molecules,
examples of substances, which are made up of atoms, are: gold, copper,
Argon and silver; and those made up of molecules includes oxygen, water
and ammonia.
- In solid,
storm’s attractive forces hold molecules together so that they are not
free to move but they can only vibrate about their mean positions. - In
liquids there are weak forces of attraction between molecules therefore
the molecules are free to move randomly. The distances between
molecules in liquids are therefore are larger than in solids.
In
case of gases the molecules experience very weak forces of attraction
and hence they are free to move randomly filling the whole space of the
containing vessel. The distances between molecules in gases are
comparatively greater than those in solids and liquids as shown in the
figure above.
case of gases the molecules experience very weak forces of attraction
and hence they are free to move randomly filling the whole space of the
containing vessel. The distances between molecules in gases are
comparatively greater than those in solids and liquids as shown in the
figure above.
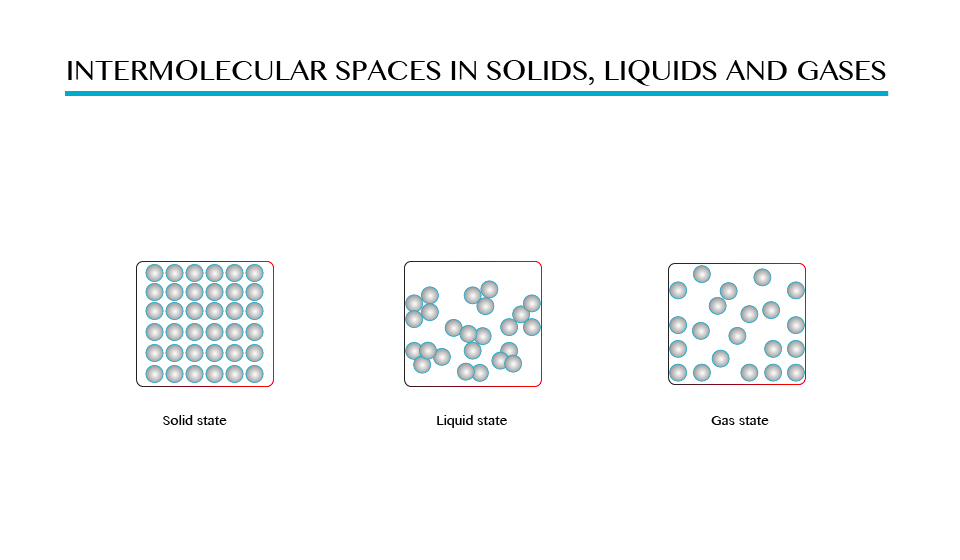
Demonstration to show the intermolecular space in solids, liquid and gases.
The Kinetic Theory of Matter
Explain the kinetic theory of matter
Generally,
when solid particles are placed in the source of lead the particles
tends to move from hot area to cold areas. These particles move because
it gains energy that called it Kinetic energy.
when solid particles are placed in the source of lead the particles
tends to move from hot area to cold areas. These particles move because
it gains energy that called it Kinetic energy.
Kinetic
theory of matter sometimes attempts to explain how properties of gases
like pressure, temperature and volume remain in constant motion.
theory of matter sometimes attempts to explain how properties of gases
like pressure, temperature and volume remain in constant motion.
There are three main parts of the Kinetic theory of matter. This includes:
- Matter is made up of tiny invisible part.
- Matter comes in different sizes.
- There is a point that the smallest particles of matter can be the fastest.
Therefore
kinetic theory of matter states, “All matter is composed of small
particles”Or “Particles of matter are in steady motion and that all
impacts between the units of matter are completely elastic”
kinetic theory of matter states, “All matter is composed of small
particles”Or “Particles of matter are in steady motion and that all
impacts between the units of matter are completely elastic”
Three States of Matter
Classify three states of matter
There are three states of matter, namely:
- Solid state
- Liquid state
- Gaseous state
Solid
state is the state of matter, which include solid materials, in which
the intermolecular force between molecules are greatest and distance
between molecules is small. Examples of solid state are wood, iron, etc.
state is the state of matter, which include solid materials, in which
the intermolecular force between molecules are greatest and distance
between molecules is small. Examples of solid state are wood, iron, etc.
Liquid
sate is the one of the state of matter in which the intermolecular
forces are low compared to solid state, there is greater distance
between one molecule and another. See on figure 1.0 (b) examples water,
soda, kerosene, and petroleum.
sate is the one of the state of matter in which the intermolecular
forces are low compared to solid state, there is greater distance
between one molecule and another. See on figure 1.0 (b) examples water,
soda, kerosene, and petroleum.
Gaseous
state is the state of matter in which there is no intermolecular forces
between molecules hence molecules are free to move from one place to
another examples of gases are hydrogen, oxygen, carbon dioxide gas.
state is the state of matter in which there is no intermolecular forces
between molecules hence molecules are free to move from one place to
another examples of gases are hydrogen, oxygen, carbon dioxide gas.
Difference between solid state, liquid state and gaseous state of matter
| Solid state | Liquid state | Gaseous state. |
| It concerns with solid matter | It concerns with liquids/ fluids matter | It concerns with gases |
| Have high intermolecular | Low intermolecular force | No intermolecular force |
| No distance between molecules | There is little distance between molecules | Molecules are far from each other |
| Good examples are iron materials, woods etc. | Good examples are water, soda, kerosene and petrol | Good examples are oxygen and hydrogen |
Rownian Movement
According to Robert Brown: Brownian
movement refers to the irregular motion of tiny particles suspended in
fluid (liquid organs). Consider the demonstrationbelow
movement refers to the irregular motion of tiny particles suspended in
fluid (liquid organs). Consider the demonstrationbelow
Robert
Brown, an English Botanist, powered some pollen grain in water and
observed that particles floating in the water were darting about.
Brown, an English Botanist, powered some pollen grain in water and
observed that particles floating in the water were darting about.
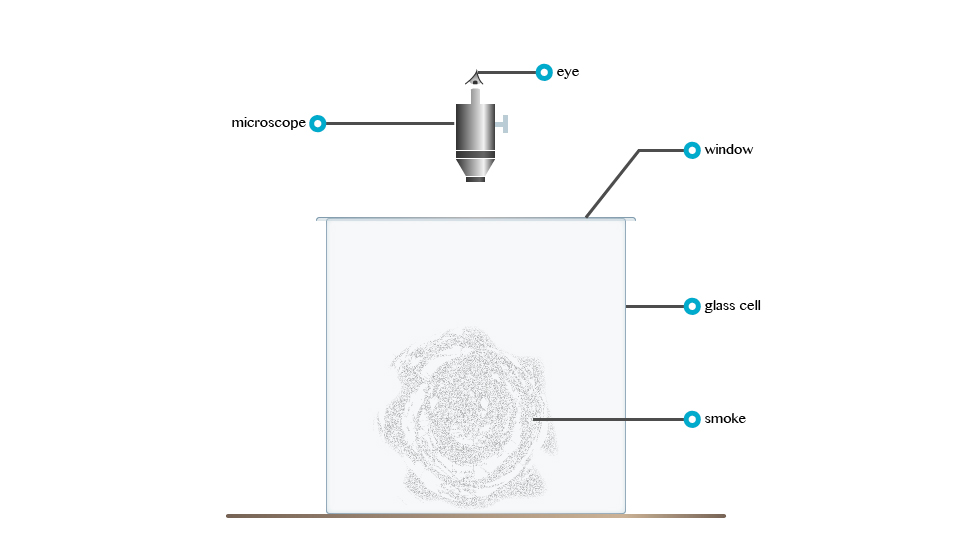
The irregular motion of tiny particles suspended in a fluid (fluid or gas) is called Brownian movement.
The tiny particles dart about because liquid molecules that are in state of motion bombard them.
Elasticity
The Concept of Elasticity
Explain the concept of elasticity
When
a force is applied to a body the dimension of the body is usually
altered. If an iron wire is stretched by small force applied to it
longitudinally, the wire returns to its original shape and size when the
force is removed.
a force is applied to a body the dimension of the body is usually
altered. If an iron wire is stretched by small force applied to it
longitudinally, the wire returns to its original shape and size when the
force is removed.
Elasticity
can be defined as the property of the iron wire by which it recovers
its original shape and size on removal of the stretching force.
can be defined as the property of the iron wire by which it recovers
its original shape and size on removal of the stretching force.
The Relationship between Tension and Extension of a Loaded Elastic Material
Justify the relationship between tension and extension of a loaded elastic material
Consider the graph below:
Point A is called the elastic limit. The straight region OA of the graph has a slope K given by the ratio.
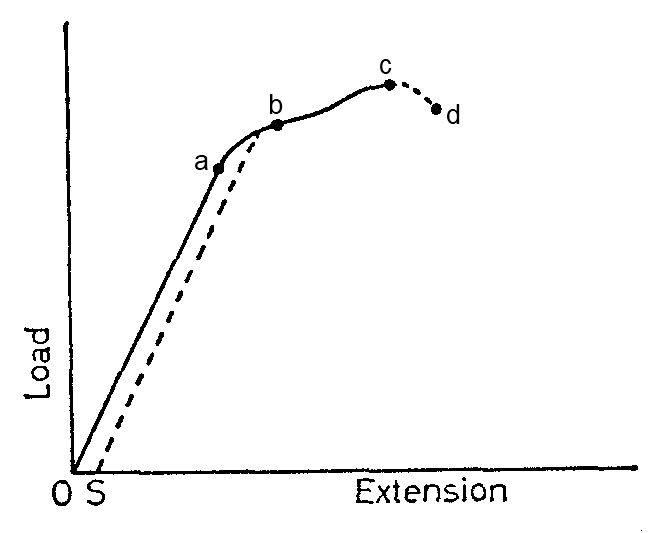
K= Tension/Extension
The ratio is called the force constant or coefficient of stiffness of the wire and it is expressed in newton per metre (N/M)
The Application of Elasticity in Real Life
Identify the applications of elasticity in real life
In
everyday life we often actually do the activities that are concerned
with the application of physics. Here are some of the application of
physics in everyday life especially in the application of Elasticity:
everyday life we often actually do the activities that are concerned
with the application of physics. Here are some of the application of
physics in everyday life especially in the application of Elasticity:
- Spring
mattress. When you sit or sleep on a spring mattress, futon style push
your weight. Pressured by the compressed spring mattress. Due to the
nature of its elasticity, stretch a spring mattress again. Spring will
be stretched and compressed, and so on. - Spring that is used as
shock absorbers on motorcycles. Springs used in the suspension systems
of motor vehicles. The purpose of this is to dampen spring a surprise
when a motorcycle driven through an uneven road surface. - Another
simple example and that you may often come across is the catapult. When
it was about to shoot birds with catapults for example, rubber
slingshots first stretch (given the gravity). Due to the nature of its
elasticity, long rubber slingshots will return to normal after a tensile
force is removed.
Adhesion and Cohesion
The Concept of Adhesion and Cohesion
Explain the concept adhesion and cohesion
Matter is made up of molecules. That exerts force of attraction. This force of attraction may be either Cohesion or Adhesion.
- Cohesion is the force of attraction between the molecules of the same substance, example water to water molecules.
- Adhesion is the force of attraction between the molecule of different substances example water to glass molecules.
Water molecules can experience the force of cohesion among themselves, where water molecules and glass molecules will experience force of adhesion.
Definite shapes of a solid are due to strong cohesion force among its molecules.
Shapes and meniscus of a liquid
When
we carried out activities involving determination of volume in a liquid
ring and measuring cylinder. The description indicated that the surface
of the liquid was carved, forming a meniscus, and that the volume must
be read at the bottom or top of the meniscus, depending on the liquid
used. For mercury, the top of the meniscus is read.
we carried out activities involving determination of volume in a liquid
ring and measuring cylinder. The description indicated that the surface
of the liquid was carved, forming a meniscus, and that the volume must
be read at the bottom or top of the meniscus, depending on the liquid
used. For mercury, the top of the meniscus is read.
The
formation of a meniscus in a liquid is due to forces of adhesion
between the liquid and the walls of the container. The adhesion of the
liquid such as water to the wall of a vessel causes an upward force on
the liquid at the edge.
formation of a meniscus in a liquid is due to forces of adhesion
between the liquid and the walls of the container. The adhesion of the
liquid such as water to the wall of a vessel causes an upward force on
the liquid at the edge.
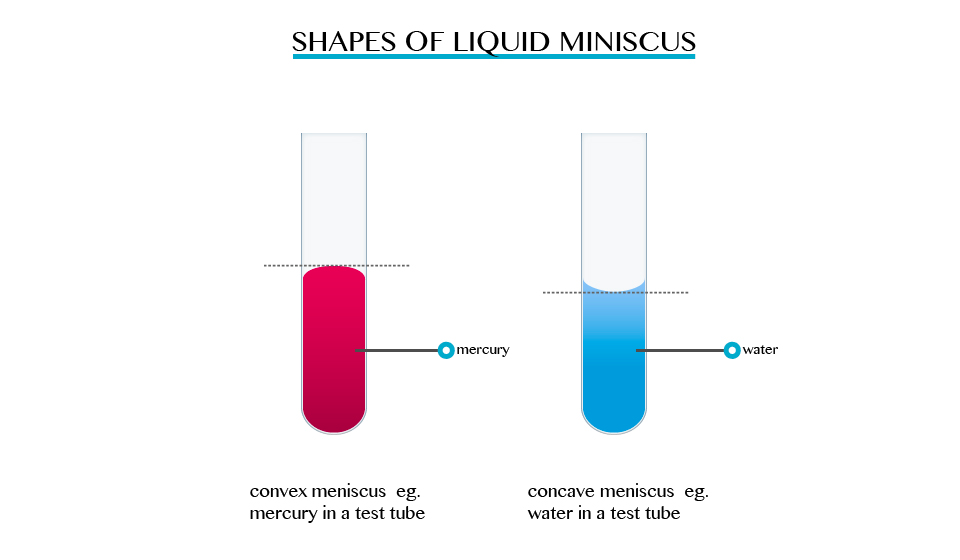
The
opposite takes place in mercury, the meniscus of water curves upwards
forming a concave shape. When a drop of each liquid, mercury and water
are placed on a glass sheet, water spreads further unlike mercury,
because of mercury’s high cohesion force among its particle.
opposite takes place in mercury, the meniscus of water curves upwards
forming a concave shape. When a drop of each liquid, mercury and water
are placed on a glass sheet, water spreads further unlike mercury,
because of mercury’s high cohesion force among its particle.
Why water wets the glass?
Why methanol does not wet the glass?
Applications of Adhesion and Cohesion in Daily Life
Identify the applications of adhesion and cohesion in daily life
Application include the following
- To stick two different objects together. Here we use the adhesive effects of tape or glue.
- Adhesion
can also be used to remove harmful materials such as bacteria from
drinking water. Adhesive forces are the source attraction substance. - Cohesion assists in transport of water in plants and animals by allowing one molecule to pull others along with it.
- The bodies of plants and animals also use the cohesion of tissue to repair damage.
- Ink sticks on paper because of adhesive force between the paper and ink.
Surface Tension
The Concept of Surface Tension
Explain the concept of surface tension
While
you may not be able to walk on water, water stride does. This is due to
the property of liquid, which is known as surface tension.
you may not be able to walk on water, water stride does. This is due to
the property of liquid, which is known as surface tension.
Surface
tension is the ability of the molecules on the surface of a liquid to
attract and stick to each other allowing them to resist an external
force. Surface tension enables insects such as water strides and
mosquitoes to walk on water. It allows small objects even metallic ones
such as needles and razor blades to float on the surface of water.
tension is the ability of the molecules on the surface of a liquid to
attract and stick to each other allowing them to resist an external
force. Surface tension enables insects such as water strides and
mosquitoes to walk on water. It allows small objects even metallic ones
such as needles and razor blades to float on the surface of water.
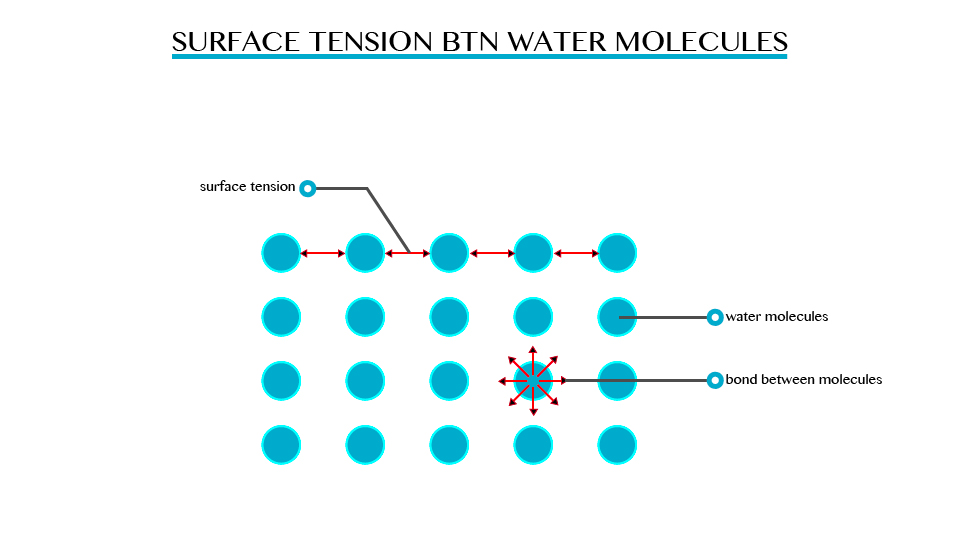
Surface
tension is a resultant attractive force between molecules in a liquid.
The molecules below the surface liquid have forces of attraction between
neighbouring particles. However molecules at the surface have no
neighbouring molecules above them. This makes them have stronger
attractive force than their nearest neighbours on the surface.
tension is a resultant attractive force between molecules in a liquid.
The molecules below the surface liquid have forces of attraction between
neighbouring particles. However molecules at the surface have no
neighbouring molecules above them. This makes them have stronger
attractive force than their nearest neighbours on the surface.
However,
when some detergent is added to water, the same objects sink to the
bottom of the trough. This means that the detergent interfered with the
surface of the liquid so decreasing the tension of the water surface.
when some detergent is added to water, the same objects sink to the
bottom of the trough. This means that the detergent interfered with the
surface of the liquid so decreasing the tension of the water surface.
Detergents are example of surfactants. A surfactant is a substance that reduces the surface tension of a liquid.
Note: the term surfactant is an aerogun for surface-active agent.
Surface tension is affected by the following
- Nature of the liquid
- Contamination/impurities
- Temperature
Application at surface tension:
- In extraction of impurities dating laboratory process.
- Surfactants are also used to make emulsion of liquid like oil and water.
- In cleaning action of soap.
Applications of Surface Tension in Daily Life
Identify the applications of surface tension in daily life
Application at surface tension
- In extraction of impurities dating laboratory process
- Surfactants are also used to make emulsion of liquid like oil and water.
- In cleaning action of soap
Capillarity
The Concept of Capillarity
Explain the concept of capillarity
This
is the tendency of a liquid to rise in narrow tubes or to be drawn into
small openings such as those between the fibres of a towel. Capillarity
can pull a column of liquid upward until the weight of liquid becomes
greater than the surface tension.
is the tendency of a liquid to rise in narrow tubes or to be drawn into
small openings such as those between the fibres of a towel. Capillarity
can pull a column of liquid upward until the weight of liquid becomes
greater than the surface tension.
In a tube, capillarity depends on the tube’s diameter but weight of water column depends on other factors besides it.
The
smaller the radius of the tube the higher the liquid will rise in it.
This implies that capillarity height is immensely proportional to the
diameter of the tube.
smaller the radius of the tube the higher the liquid will rise in it.
This implies that capillarity height is immensely proportional to the
diameter of the tube.
By definition
Capillarity
is defined as the tendency of liquid to rise in narrow tubes or to be
drawn into small openings such as those between the fibres of a towel.
is defined as the tendency of liquid to rise in narrow tubes or to be
drawn into small openings such as those between the fibres of a towel.
Capillarity action is the ability of a liquid to raise or fall in a narrow tube.
Note:
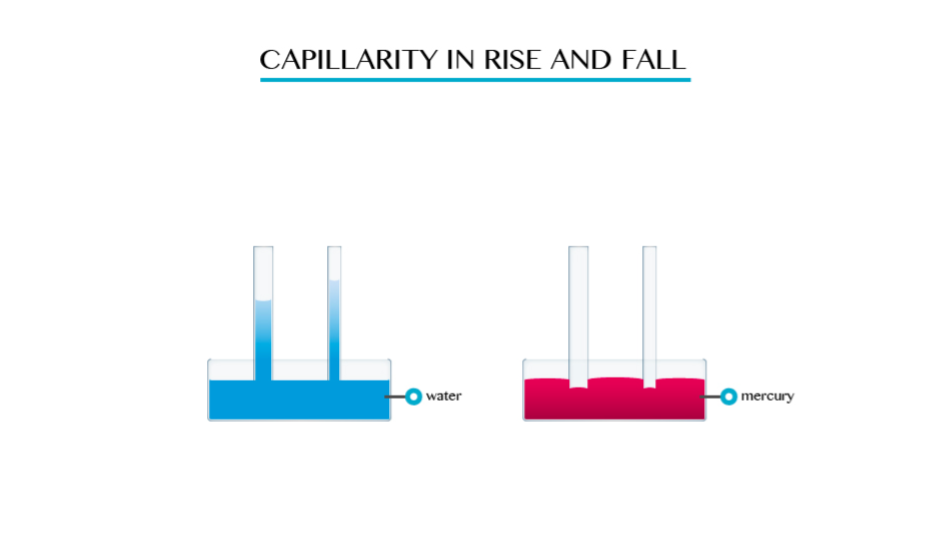
- Capillarity
depends on the type of liquid. For example if you dip capillarity tube
in water the water rises in the tube and above the level of the water in
the vessel. - If the tube is dipped in mercury, the liquid does not rise in the tube. It suffers capillarity depression.
Applications of Capillarity in Daily Life
Identify the applications of capillarity in daily life
The application includes:
- Capillarity is essential to plants and animals.
- In
plants, it facilitates the transport of water and nutrients from the
roots to the leaves where photosynthesis produces the plants food. In
animals it assists in the circulation of blood. - Capillarity promotes the movement of ground water.
- It is the principles on which paper and fabric towels work to absorb water.
- Cotton clothing in hot climates uses capillarity action to draw perspiration away from the body.
- In an oil or kerosene lamp capillarity draws the fuel up into the wicker where it can be burnt.
- A writing Rubin splits in the middle so that a fine capillary is formed.
Osmosis
The Concept of Osmosis
Explain the concept of osmosis
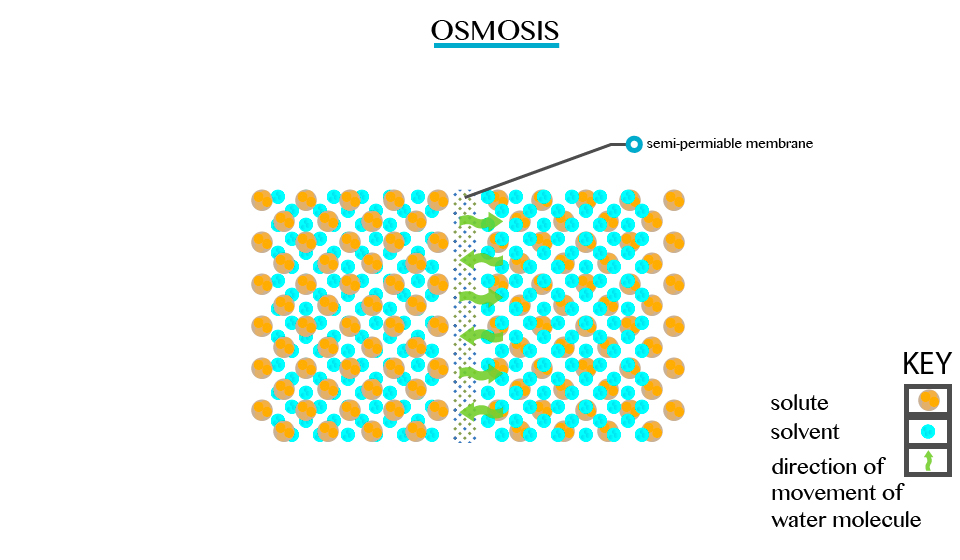
Defined as the movement of a solvent from a region of low concentration through semi permeable membrane.
Particles
will diffuse through the membrane in an attempt to equalize the
concentration on either side. E.g. two solutions of different
concentration separated by a semi permeable membrane. The membrane is
permeable to the smaller solvent molecules but not to the larger solute
molecules. Osmosis stops when the concentration becomes the same on
either side of the membrane.
will diffuse through the membrane in an attempt to equalize the
concentration on either side. E.g. two solutions of different
concentration separated by a semi permeable membrane. The membrane is
permeable to the smaller solvent molecules but not to the larger solute
molecules. Osmosis stops when the concentration becomes the same on
either side of the membrane.
Osmosis stops when the concentration becomes the same on either side of the membrane.
Applications of Osmosis in Daily Life
Identify the applications of osmosis in daily life
Applications of osmosis in daily life:
- Control the movement of water and nutrients in and out of the cell.
- Filtration processes.
- Removal of harmful ingredients from dinking water.
- Removing salt from seawater so as to make it suitable for drinking and for other domestic uses.