Cell Differentiation and Organic Constituent of the Cells
In this article, are you looking for CYTOLOGY: Cell Differentiation And Organic Constituent Of The Cells – Biology Notes Form 5 & 6
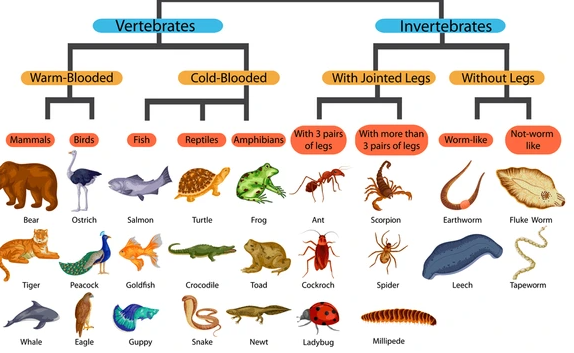
This is the specialization of a cell in terms of both structure and functions.
Ability of a cell to perform single function is called cell specialization.
ORGANIC CONSTITUENT OF THE CELLS
-It is a great unifying theme in biology.
It finds applications in fields like;
- Agriculture; in developing pesticides and herbicides.
- Medicine; including all pharmaceuticals.
- Fermentation; baking products, food products and breweries.
- New development of biology eg genetic engineering.
ELEMENTS FOUND IN LIVING ORGANISMS ARE
- Chief/ macro elements: hydrogen (H), carbon(C), nitrogen (N), oxygen (O), phosphorous (P), sulphur(S).
- Ions – sodium(Na+) , magnesium (Mg2+) , chlorine( cl–) , calcium (Ca2+) etc.
- Trace elements – manganese(Mn) , iron(Fe) , cobalt(Co),copper (Cu) , molybdenum(Mo) and iodine(I).
MACROMOLECULE(S)
Macromolecule is a giant molecule made from many repeating units. The molecules built are polymers and the individual units are monomers.
-The units are joined together by a chemical process called CONDENSATION which means removal of water.
-The units can be broken down again by an opposite process known as hydrolysis which means adding of water.
The most important macromolecules in biology are;
- Polysaccharides( carbohydrates)
- Protein
- Lipids
- Nucleic acids.
And their constituent monomers are; monosaccharide’s, amino acids, glycerol, fatty acids and nucleotides respectively.
Others are;
- Adenosine triphosphate (ATP).
ORGANIC SUBSTANCES ( CHEMICAL NATURE AND IMPORTANCE)
1. CARBOHYDRATES
They are substances which contain carbon, hydrogen and oxygen with the general formula of (CHO)n where n is a real number.
Characteristics of carbohydrates.
They are either simple sugars or compound sugars.
The compound sugars are formed by condensation of simple `sugar molecules.
- They are hydrate of carbon from the proportion of hydrogen and oxygen in water.
- The basic carbohydrate unit is thus a sugar which is the derivative of a poly hydrosol alcohol.
Alcohol is the paraffin compound with hydrogen atom replaced by the univalent hydroxyl (OH) group.
The simpler hydroxyls are the glycol and glycerol and the simplest of sugar is the glycerose (glycerin).
The carbohydrate contains several hydroxyl groups.
4. Some contain aldehyde (-CHO) group and others contain ketone group ( -CO-)
Examples;
- Glucose: is a pentahydroxyl alcohol with the aldehyde group.
GLUCOSE
2. Fructose: is the pent hydroxyl alcohol with ketone group.
Complex sugars are built from the basic sugar units called monosaccharides through the process of condensation polymerization.
Many sugars are reducing sugars and others are non-reducing sugars but give rise to reducing sugars on hydrolysis with enzymes or mineral acid (mostly dilute HCL)
NB: Carbohydrates are called reducing sugar because they act as reducing agents supplying electrons from their functional groups i.e. the aldehyde and ketone groups which can reduce the cu2+ ions to cu+ ions which appear orange or yellow ppt (precipitate).
The true carbohydrates are saccharides with a combination of sugar units. These are divided into three main classes
- monosaccharides – with a single sugar unit
- Disaccharides – with two sugar units.
- Polysaccharides- with many sugar units.
SUGAR
Sugar which include mono and disaccharides are all soluble in water. They have a sweet taste.
They are crystalline and small molecules.
Those with a potentially active aldehyde or ketone group are the reducing sugars e.g. glucose.
| sugar | Natural occurrence |
| Glucose | Plant juice and grape sugar |
| Galactose | From fruits. |
| Fructose | From fruits |
| Maltose | From germinating seeds ( cereals) |
| Sacrose | From sugar cane ( in plants) |
| Lactose | From milk |
Sugars without potentially active reducing groups are known as non-reducing sugars e.g.
Monosaccharides
- Have general formula (CnH2nOn)
- All are reducing sugars
- They are classified according to the number of carbon atoms e.g.;
Trioses have 3 carbon atoms
Tetraoses have 4 carbon atoms
Pentoses have 5 carbon atoms
Hexoses have 6 carbon atoms
Heptoses have 7 carbon atoms
-Of code, hexoses and pentoses are most common and triose being the true sugar.
-Pentose sugars are never occurring but only in combination with other groups Of compounds.
Riboses- this occurs in one kind of nucleic acid. A derivative of deoxyribose
Hexose. The most important free sugar.
D-glucose
D- Fructose these are the most common sugars.
Structure of Monosaccharides
Glucose in common with other hexoses and pentoses easily forms stable ring structure. At any one time most molecules oxists as rings rather than
In case of glucose carbon atom number 1 may combine with the oxygen atom an carbon 5. This form a six -sided structure known as a pyranose ring
In case of fructose, carbon atom number 2 links with the oxygen an carbon atom number. This form a five sided structure known as furanose ring Both glucose and fructose can exist in Beth pyranose ring.
In case of fructose, carbon atom number 2 links with the oxygen on number 5. This form a five sides structure known as furanose ring Both glucose and fructose can exist in both pyranose and furanose and furanose ring form.
Cell Differentiation And Organic Constituent Of The Cells
STRUCTURE PG 13 UB
Furanose
Most carbohydrate in common glucose can exists as a numbee of isomers (they posses the same molecular formula but differ in the arrangement of this atoms).
One type of isomer called stereoisomerism. occurs when the atom, or group, are joined together but differ in Their arrangement in space one form of stereoisomer is called Optical Isomersm, result in isomer which can rotate the plane of polarized light.
If the substance rotates the plane of polarisation to the right it is said to be dexTro-rotatory (d) and if to the left is laevo-rotatory (L) Optical isomerism is a property of any compound which can exist in two forms whose structure are minor image. Like right and left handed gloves
Example.
L-Form isomer mirror. D-form isomer
Functions of monosaccharides.
- PENTOSES (C5H10O5 ) e.g. ribose, deoxyribose, ribulose.
- Synthesis of nucleic acids e.g. Ribose is the chief constituent of the RNA.
- Synthesis of co-enzymes e.g. ribose synthesis (NAD and NADP)
HEXOSES (C6H12O6) e.g. fructose, glucose, galactose.
- Sources of energy when oxidized by respiration.
- Synthesis of disaccharides.
- Synthesis of polysaccharides.
TRIOSES (C3H6O3) e.g. glyceraldehydes, dihydroxyacetone.
- Intermediate in respiration (glycolysis).
- Photosynthesis (dark reaction) RUBP as an acceptor of CO2
- Carbohydrate metabolism.
Disaccharides
*Disaccharides are formed by the condensation or polymerlization of two monosaccharides.
The most common disaccharides are;
- Maltose = glucose + glucose
- Lactose = glucose + galactose
- Sucrose = glucose + fructose.
In reducing sugars e.g. Lactose and maltose, one of the hexose residue retains its
aldehyde or ketone groups as an intact unit as reducing sugar.
Maltose is a disaccharide produced upon incomplete hydrolysis of the polysaccharide starch.
-It is found in germinating seeds.
-It is also produced commercially for use in production of beer.
-Maltose is produced of two D-glucose units joined by a α-glycosidic bond between the anomeric carbon of one glucose unit and the number 4 carbon of the other glucose unit.
This specific bond formed an α-1,4-glycosidic bond also found in starch and glycogen.
NB: The numeric hydroxyl group of one of the glucose units participates in the glycosidic bond and
Therefore cannot be easily oxidized.
However the anumeric hydroxyl of the other glucose unit is not as occupied and this glucose unit exists in the equilibrium with free aldehyde solution.
Thus maltose is oxidized by Fehling’s solution, benedict’s solution or any other suitable reagent.
Lactose
Constitutes some 3% to 5% of the milk of animal including cows and humans.
This disaccharide is composed of one galactose unit and one glucose unit joined by a glycosidic bond between the anomer of galactose and the number 4 carbon of glucose. A β-1, 4 –glycosidic bond.
Glucose unit of the lactose still exists as an equilibrium mixture of α and β anomers and the free aldehyde in solution. Lactose is thus a reducing sugar.
Sucrose
-It is found in fruits and vegetables.
-Sugarcane and sugar beets are the commercial sources and used as table sugar.
-Sucrose is composed of one fructose unit joined by two glycosidic bonds.
Sucrose is not a reducing sugar since both anomeric carbons participates in the glycosidic bonds and thus no free aldehyde or ketone exists in solution.
NB: D is the hydroxyl group attached to the anomeric carbon atom (the anomeric hydroxyl group) is drawn on the same side of the ring as the last -CH2OH group for the β-anomer and the opposite side of the ring for the α-anomer.
The D-galactose only differs from the D-glucose only in the orientation of the groups bonded to Carbon no. 4. Ingested D-glucose (from milk and some other complex polysaccharide) is normally converted to D glucose in the human body.
The inability to perform this ionization (conversion of one isomer to another) results in a disease called galactosemis.
POLYSACCHARIDES
-Have high molecular weight formed by condensation of large number of monosaccharide units.
They include;
- Starch, glycogen, cellulose, chitin and insulin.
-All polysaccharides are insoluble in water forming colloidal solution.
-They are non –reducing sugars.
-They are Non- crystalline and as structural materials e.g. Cellulose.
-Represented by chemical formula ( C5H10O5) n or (C6H10O5)n where n is a whole number ranging from 300-400.
Starch
Usually occur in a white powder-form at room temperature.
It forms a solution with hot water and a gel on cooling.
- During digestion, it is converted to a mixture of detrins. Later from maltose to glucose units.
- It is largely stored in plants and it is a result of photosynthesis.
- In plants, starch is found in the storage parts such as roots and stem tubers, corn and some rhizomes.
A starch granule is composed of
- A core of amylase
- Amylopeptin
- Amyloplast membrane.
Diagrams of amylase and amylopectin.
NB: Amylase and amylopectin are two different forms of starch.
- Both have linear chains of glucose units joined by α-1, 4-glycosidic bond.
Amylase is linear polymer of α-1, 4-glucose unit and is insoluble in water.
Amylopectin is a branched polymer; the main chain of amylopectin is joined by α-1,4-glycosidic bond as in amylase. However about every 20-30 glucose units there are branches joined by α-1,6-glycosidic bond.
Amylopectin is not soluble in water.
When boiled to about 2000C starch is partially hydrolyzed to a mixture of dextrins. However, when heated with dilute mineral acids, starch is hydrolyzed via dextrin to glucose.
-In living things (tissues) the hydrolysis is of the following sequence.
-A suspension of glucose gives a blue black coloration with iodine.
-Amylopectin is compact as it has many branches of 1, 6- glycosidic bonds.
Biological importance
- Storage of food in seeds, example; cereals and legumes
- Important for human food.
GLYCOGEN
-This is called animal starch formed by condensation polymerization of glucose on units.
-It is very similar to amylopectin in structure.
-It is a polymer of glucose units joined by α-1,4- bonds and with α-1,6- bonded branches . it is a white soluble powder and non reducing sugar.
OCCURRENCE
- Mainly in vertebrates liver and muscles.
- Also some maize seeds and fungi.
Glycogen differs from amylopectin because it is more highly branched than amylopectin with one branch point about every 8 and 12 glucose units.
Biological advantage.
- It is important food storage in muscle and liver of vertebrates and fungi.
- It provides energy and is an energy substance.
CELLULOSE
This is an important structural material in plants.
It largely constitutes the chemistry of the cell wall.
-Chemically cellulose is composed of several thousands of glucose units joined together by 1, 4- glycosidic bonds. The units are so arranged that the bonds alternate in appearance.
-This lead to the cross links of hydrogen bonds between the parallel running cellulose molecules.
As a result of this, cellulose becomes tough with very high tensile strength.
The shape of a cellulose fibre is of the nature below.
Chemical structure of cellulose is presented as follows.
Hydrolysis of cellulose.
In the living tissues, hydrolysis of cellulose takes place in two stages;
Commercial use of cellulose
It is a raw material in the manufacture of many industrial products such as papers, rayon and plastics.
The rayon made from cellulose are used in the manufacture of industrial belts and tyre cords.
Cellulose derivatives such as cellulose nitrate are used in the manufacture of films.
Cotton, a pure form of cellulose is used in the manufacture of clothes.
CHITIN
This is closely related to cellulose in structure and function, being a structural polysaccharide.
-Structurally it is identical to cellulose except that the OH group at carbon 2 is replaced by – NH.CO. NH3.
-This is a result of amino sugar (glucosamine) combine with acetyl group.
Chitin is therefore a polymer of N-acetylglucoamine.
Inulin
This is another storage carbohydrate which largely occurs of many plants.
It also occurs in small quantities in many monocots. Hydrolysis by dilute mineral acids or specific enzymes e.g. inulin produce fructose only.
It is a polymer of fructose molecule.
Inulin inulase fructose
Summary roles of carbohydrates.
1. They built up a cell plasma membrane. It is made up of carbohydrates and so they are used to build up the body of a living organism.
2. They are used as a substrate in respiration (to produce energy) as raw materials. Glucose is the base raw material in glycolysis.
3. Are useful in pollination. Nectar which attracts pollinators is made up of sugars.
4. Are useful in storage purpose for future metabolism eg starch, glycogen and laminarin.
5. Used in the balance of osmotic pressure as they make solutes in the blood.
6. Are used in inheritance and control of the body activities as they make the genes e.g. deoxyribose of DNA and ribose of RNA are pentose sugars.
*Other uses are from the above discussions.