Thermal Energy
The Concept of Thermal Expansion of Gases
Explain the concept of thermal expansion of gases
Gases
expand when heated just like solids and liquids. This is because the
average kinetic energy of the molecules in a gas is directly
proportional to the absolute temperature of the gas. Heating the gas
increases the kinetic energy of its molecules, making them vibrate more
vigorously and occupy more space.
expand when heated just like solids and liquids. This is because the
average kinetic energy of the molecules in a gas is directly
proportional to the absolute temperature of the gas. Heating the gas
increases the kinetic energy of its molecules, making them vibrate more
vigorously and occupy more space.
The Relationship between Volume and Temperature of Fixed Mass of Air at Constant Pressure
Investigate the relationship between volume and temperature of fixed mass of air at constant pressure
Three
properties are important when studying the expansion of gases. These
are; pressure, volume and temperature. Charles law states that the
volume of a fixed mass of gas is directly proportional to the absolute
(Kelvin) temperature provided the pressure remains constant.
Mathematically V1T2 = V2T1.
properties are important when studying the expansion of gases. These
are; pressure, volume and temperature. Charles law states that the
volume of a fixed mass of gas is directly proportional to the absolute
(Kelvin) temperature provided the pressure remains constant.
Mathematically V1T2 = V2T1.
Example 2
the
volume of gas at the start is recorded as 30 cm3with a temperature of
30°C. The cylinder is heated further till the thermometer records 60°C.
What is the volume of gas?
volume of gas at the start is recorded as 30 cm3with a temperature of
30°C. The cylinder is heated further till the thermometer records 60°C.
What is the volume of gas?
Solution:
We know,V/T = constant
therefore,
V1/T1=V2/T2
V1 =30 cm3
T1 =30°C = 30+273 = 303K(remember to convert from Celsius to Kelvin)
T2 =60°C = 60+273 = 333K
V2 =?
V1/T1=V2/T2
V2=V1xT2/T1
V2=30×333/303
= 32.97 cm3
The Relationship between Pressure and Volume of a Fixed Mass of Air at Constant Temperature
Investigate the relationship between pressure and volume of a fixed mass of air at constant temperature
The
relationship obtained when the temperature of a gas is held constant
while the volume and pressure are varied is known as Boyle’s law.
Mathematically, P1V1 = P2V2. Boyle’s law states that the volume of a
fixed mass of gas is inversely proportional to its pressure if the
temperature is kept constant.
relationship obtained when the temperature of a gas is held constant
while the volume and pressure are varied is known as Boyle’s law.
Mathematically, P1V1 = P2V2. Boyle’s law states that the volume of a
fixed mass of gas is inversely proportional to its pressure if the
temperature is kept constant.
PressurexVolume = constant
pxV = constant
Example 3
The volume of gas at the start is 50 cm3with a pressure of 1.2 x 105Pascals. The piston is pushed slowly into the syringe until the pressure on the gauge reads 2.0 x 105Pascals. What is the volume of gas?
Solution:
We know
p x V = constant
therefore,
p1xV1= p2xV2
p1=1.2 x 105Pascals
V1=50 cm3
p2=2.0 x 105Pascals
V2=?
p1xV1= p2xV2
V2=p1xV1/p2
V2=1.2×105x50/2.0 x 105
V2= 30 cm3
The Relationship between Pressure and Temperature of a Fixed Mass of Air at Constant Volume
Investigate the relationship between pressure and temperature of a fixed mass of air at constant volume
To
investigate the relationship between the pressure and the temperature
of a fixed mass, the volume of the gas is kept constant. The pressure is
then measured as the temperature is varied. P1/T1 = P2/T2 ,this is
called pressure law. The pressure law states that the pressure of a
fixed mass of a gas is directly proportional to the absolute temperature
if the volume is kept constant
investigate the relationship between the pressure and the temperature
of a fixed mass, the volume of the gas is kept constant. The pressure is
then measured as the temperature is varied. P1/T1 = P2/T2 ,this is
called pressure law. The pressure law states that the pressure of a
fixed mass of a gas is directly proportional to the absolute temperature
if the volume is kept constant
Example 4
Pressure of gas is recorded as 1.0 x 105N/m2at a temperature of 0°C. The cylinder is heated further till the thermometer records 150°C. What is the pressure of the gas?
Solution:
We know,p/T = constant
therefore,
p1/T1= p2/T2
p1=1.0 x 105N/m2
T1=0°C = 0+273 = 273K(remember to convert from Celsius to Kelvin)
T2=150°C = 150+273 = 423K
p2 =?
p1/T1= p2/T2
p2=p1xT2/T1
p2=1.0×105x423/273
= 1.54 x 105N/m2
The General Gas Equation from the Gas Laws
Identify the general gas equation from the gas laws
The three gas laws give the following equations:
- pV = constant(when T is kept constant)
- V/T = constant(when p is kept constant)
- P/T= constant(when V is kept constant)
These 3 equations are combined to give the ideal gas equation:
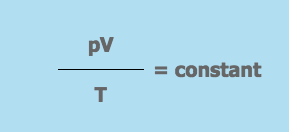
Where,
- p = the pressure of the gas
- V = the volume the gas occupies
- T = the gas temperature on the Kelvin scale
From
this equation we know that if a fix mass of gas has starting values of
p1, V1 and T1, and then some time later has value p2, V2 and T2, the
equation can be written as:
this equation we know that if a fix mass of gas has starting values of
p1, V1 and T1, and then some time later has value p2, V2 and T2, the
equation can be written as:
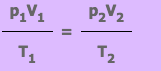
Exercise 1
Sabah
pumps up her front bicycle tyre to 1.7 x 105Pa. The volume of air in
the tyre at this pressure is 300 cm3. She takes her bike for a long ride
during which the temperature of the air in the tyre increases from 20°C
to 30°C. Calculate the new front tyre pressure assuming the tyre had no
leaks and so the volume remained constant?
pumps up her front bicycle tyre to 1.7 x 105Pa. The volume of air in
the tyre at this pressure is 300 cm3. She takes her bike for a long ride
during which the temperature of the air in the tyre increases from 20°C
to 30°C. Calculate the new front tyre pressure assuming the tyre had no
leaks and so the volume remained constant?
Absolute Scale of Temperature
Explain absolute scale of temperature
Absolute
zero is the lowest temperature that can be attained theoretically. It
is not possible to attain this temperature because all gases liquefy
before attaining it. The kelvin scale of temperature is obtained by
shifting the vertical axis to -273 degrees Celsius and renaming it 0 K.
On the scale 0 degrees Celsius becomes 273 K and 100 degrees Celsius
corresponds with 373 K.
zero is the lowest temperature that can be attained theoretically. It
is not possible to attain this temperature because all gases liquefy
before attaining it. The kelvin scale of temperature is obtained by
shifting the vertical axis to -273 degrees Celsius and renaming it 0 K.
On the scale 0 degrees Celsius becomes 273 K and 100 degrees Celsius
corresponds with 373 K.
Convertion of Temperature in Degrees Centigrade (Celsius) to Kelvin
Convert temperature in degrees centigrade (celsius) to kelvin
The
Kelvin temperature scale takes its name after Lord Kelvin who developed
it in the mid 1800s. It takes absolute zero as the starting point and
temperature measurements are given the symbol K (which stands for
“Kelvin”). Temperature differences on the Kelvin scale are no different
to those on the Celsius (°C) scale. The two scales differ in their
starting points. Thus, 0°C is 273K.
Kelvin temperature scale takes its name after Lord Kelvin who developed
it in the mid 1800s. It takes absolute zero as the starting point and
temperature measurements are given the symbol K (which stands for
“Kelvin”). Temperature differences on the Kelvin scale are no different
to those on the Celsius (°C) scale. The two scales differ in their
starting points. Thus, 0°C is 273K.
Converting from Celsius to Kelvin
- Temperature in °C + 273 = Temperature in K
Converting from Kelvin to Celsius
- Temperature in K – 273 = Temperature in °C
Example 5
The temperature of a gas is 65 degrees Celsius. Change it to the kelvin scale.
Solution
T(K) = degrees Celsius + 273, T(K) = 65+273
therefore T(K) = 338 K.
Standard Temperature and Pressure (S.T.P)
Explain standard temperature and pressure (S.T.P)
The
standard temperature and pressure (S.T.P) is a set of conditions for
experimental measurements to enable comparisons to be made between sets
of data. The standard temperature is 0 degrees Celsius (273 K) while the
standard pressure is 1 atmosphere (101300 Pa or 760 mm of mercury).
standard temperature and pressure (S.T.P) is a set of conditions for
experimental measurements to enable comparisons to be made between sets
of data. The standard temperature is 0 degrees Celsius (273 K) while the
standard pressure is 1 atmosphere (101300 Pa or 760 mm of mercury).
Expansion of Gas in Daily Life
Apply expansion of gas in daily life
Land
and sea breezes are the result of expansion of air caused by unequal
heating and cooling of adjacent land and sea surfaces. The piston engine
and firing bullets from guns work under principles of expansion of
gases.
and sea breezes are the result of expansion of air caused by unequal
heating and cooling of adjacent land and sea surfaces. The piston engine
and firing bullets from guns work under principles of expansion of
gases.






















































Thanks so much for the article post. Really Cool.
It’s awesome to visit this site and reading the views of all friends concerning this paragraph, while I am also keen of getting experience.
I cannot thank you enough for the article post. Really looking forward to read more. Fantastic.
Really appreciate you sharing this post. Really looking forward to read more. Will read on…
Say, you got a nice blog article. Really thank you! Really Great.